Decision: Mylan Pharm. Inc. v. Research Corp. Techs., Inc., Nos. 2017-2088, 2017-2089, 2017-2091 (Fed. Cir. Feb. 1, 2019).
Background
In November 2015, Argentum petitioned for inter partes review of U.S. Reissue Patent 38,551 ("the '551 patent"), owned by Research Corporation Technologies, Inc. ("RCT"), asserting eight grounds of invalidity. The '551 patent is directed to compounds and pharmaceutical compositions for treating epilepsy and other central nervous system disorders. The Patent Trial and Appeal Board ("Board") instituted review on two obviousness grounds.
After institution, Mylan, Breckenridge, and Alembic, each of whom had been sued for infringement of the '551 patent over one year prior, filed petitions for review and motions for joinder. The Board instituted these additional petitions and joined each with the Argentum IPR, but limited Mylan, Breckenridge, and Alembic's involvement to an "understudy" role that was bound to the evidence and arguments in Argentum's petition.
Before the Board, Argentum proffered a structural obviousness analysis, relying on a 1991 article for the disclosure of its most potent compound ("compound 31") as the "lead" compound. It also relied on a drug discovery book chapter for motivation to modify compound 31 by replacing the amine (–NH–) of its methoxyimino group with a methylene (–CH2–) link. Among other reasons, Argentum argued that "a person of skill in the art would have been motivated to substitute the –NH– group in 3l for a –CH2– group because it is a more common and acceptable moiety for pharmaceutically active compounds." This proposed modification of compound 31 would lead to lacosamide, as claimed by the '551 patent:
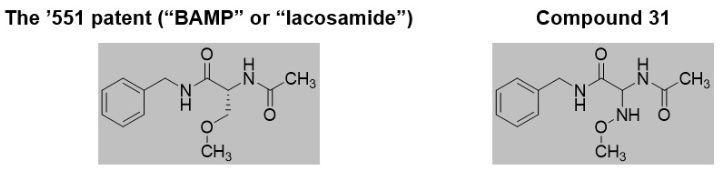
In its Final Written Decision, however, the Board found that no challenged claim had been proven unpatentable. While the Board assumed arguendo that compound 31 would be a lead compound, it found that a POSA would not have been motivated to make the proposed modification. The Board found, among other things, that (1) the primary reference taught that the compounds without the –NH– (or other features not found in compound 31) had reduced activity and (2) the proposed modification would have led to significantly different biological activity.
Of the four petitioners, only the three joined petitioners (Mylan, Breckenridge, and Alembic) appealed to the Federal Circuit.
Issues: (1) Do appellants, who were joined as petitioners more than one year after being sued, have standing to appeal where the original petitioner lacked standing? (2) Was the Board's determination that there was no motivation to modify the alleged lead compound supported by substantial evidence?
Outcome: Affirmed (3-0).
The Federal Circuit first confirmed that appellants possessed standing for the appeal by examining the use of the term "parties" in both 35 U.S.C. §§ 315(c) and 319. Specifically, the Court recognized that appellants had been joined as petitioners, or "parties", to the IPR under § 315(c); and then noted that under § 319, "[a]ny party to the inter partes review" has the right to be a party to the appeal. Prohibiting appellants from appealing would, in the Federal Circuit's view, require different interpretations of "party" between these two statutory sections. The Court also rejected RTC's argument that allowing this appeal by the joined petitioners would constitute an "end-run around the statutory time-limit for instituting IPR proceedings" and noted that RTC had provided no support for its policy argument that the joined petitioners should not have standing independent of the original petitioner.
Turning to the merits of the appeal, the Federal Circuit, like the Board, assumed arguendo that compound 31 would have been a suitable lead compound. Examining the proffered motivation to modify compound 31, the Court found that appellants had failed to establish motivation to remove the –NH– amine group because, as found by the Board based on the teachings of the primary reference and credited expert testimony, a POSA would have expected (1) reduced potency and (2) significant conformational changes that could alter biological activity.
Lastly, the Court denied appellants' alternative request that the case be remanded in light of the Supreme Court's decision in SAS Institute, Inc. v. Iancu, finding that appellants had waived this request by raising it for the first time in their rebuttal during oral argument. Although SAS was decided after briefing was complete, the Court noted that the decision had issued over six months earlier, and that appellants could have raised it in a citation to supplemental authority or even during their opening oral argument. By raising it during their rebuttal argument, RTC had no opportunity to respond, and given the circumstances of the case, the Federal Circuit found this constituted waiver.
Prosecution Takeaway:
Parties joined as petitioners to IPR proceedings have standing to appeal PTAB decisions to the Federal Circuit, even if their claims would have been traditionally time-barred due to prior litigation and even if the original petitioner lacks appellate standing.
The lead compound analysis is a fact-intensive inquiry.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


