On 6 June 2016, China Food and Drug Administration ("CFDA") published Administrative Measures on Product Formula Registration of Infant Formula Milk Powder ("Measures") for purpose of further monitoring enterprises to design formula scientifically and enhance R&D, production as well as testing capacity to ensure high quality of infant formula milk powder. The Measures will take into force on 1 October 2016.
The Measures applies to both domestic infant formula milk powder manufacturers and oversea manufacturers exporting infant formula milk powder products to China.
The Measures is deemed as a major step in China's reform of its infant formula industry. One purpose of the Measures is to restrict the number of brands and recipes. China has 103 licensed infant formula milk powder manufacturers which market around 2,000 different products. The large product portfolio, differential labeling of identical formulations and region specific marketing of these products has made it a nightmare for Chinese consumers and regulators. By implementing the extremely strict new rule, China will push infant formula companies to develop their R&D ability, upgrade production conditions and promote product heterogeneity.
Applicant
Approved domestic and foreign infant formula milk powder manufacturers should be the qualified applicant for applying for registration of infant formula recipes with CFDA. For foreign manufacturers, for the sake of convenience, they are allowed to file the application through an agent in China.
Key Materials required for Registration
For purpose of registration of infant formula recipes with CFDA, the following materials shall be prepared and submitted accordingly:
- Application form;
- Business License of the applicant;
- Quality safety standards for raw and auxiliary materials;
- R&D report for IF recipe;
- Production process description;
- Test report;
- Supporting documents for proving R&D, production and
testing abilities;
- Other supporting documents for demonstrating the science and safety of the recipe;
3 Series, 9 Formulations
Some domestic enterprises like to promote several different "new formula" brands with very similar formula, which has mislead the consumers and created chaos in the market. In order to solve this problem, the Measures regulates that each enterprise shall not have more than 3 formula series with 9 recipes in principle.
In addition, there must be significant difference between recipes for the same stage applied by the same company, supported by solid scientific evidence. Every manufacturer can only hold 3 series and 9 recipes at most. Every series should include infant formula (0-6 months, stage 1), formula for elder infants (6-12 months, stage 2) and formula for young children stage 1 (12-36 months, stage 3).
Wholly-owned subsidiaries who have already had approved infant formula recipes and been licensed for production are allowed to use other recipes registered by other wholly-owned subsidiaries under the same group company.
On-site Inspection
To examine the application materials, CFDA need to investigate the application material, as well as the consistence of the claimed recipes and the real content of the infant formula. For that purpose, CFDA will inform the applicant for on-site checking and sample testing.
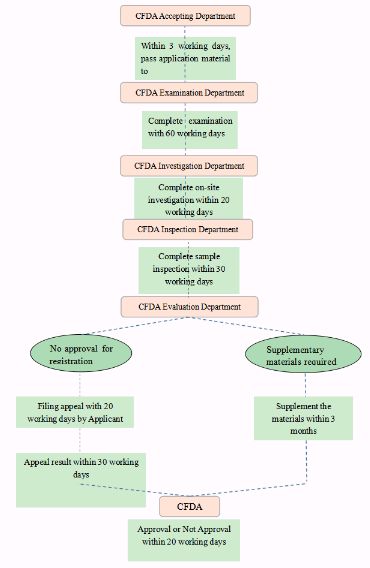
General Process and Time required for Registration of Infant Formula Recipes
Label and Specification
The Measures requires applicants to submit label and specification samples, explanations and certification materials of claim in the specification when they apply for infant formula registration. The content of the formula as contained in the label and specification shall be consistent with the approved formula by CFDA. In addition, for foreign applicant, the claimed source of lactogenesis or raw milk powder should be indicated clearly. Vague indication such as "imported milk collection", "from overseas pasture", "ecologic pasture", "imported raw materials" is forbidden.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.
