On May 18, 2018, the Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, released final guideline for implementation of provisions of the Public Procurement (Preference to Make in India) Order (PPO), 2017, with respect to public procurement of goods and services in medical devices1.
The Department of Industrial Policy and Promotion (DIPP) issued PPO, 2017, and to facilitate the implementation of the order it had identified DoP as the Nodal Ministry for implementing certain provisions of the PPO 2017 related to medical devices product category. i.e.
- Para 3 of PPO, 2017, makes it mandatory for procuring entities to give purchase preference to local suppliers,
- Para 5 of PPO, 2017, empowers Nodal Ministry to prescribe percentage and the manner of calculation of minimum local content in respect of any particular item relating to Pharmaceutical sector, and
- Para 9 of PPO, 2017, deals with verification of local content.
Now DoP has issued the following guidelines with respect to public procurement of good and services in Medical Devices under PPO, 2017-
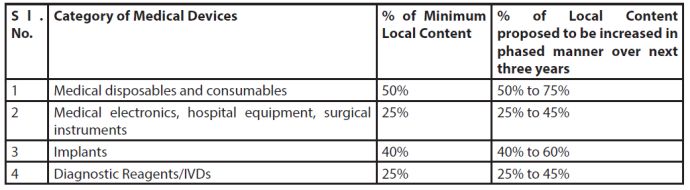
Percentage of Minimum Local Content
Medical Device Industry (MDI) is a multiproduct industry responsible for provisioning of wide variety of crucial medical products ranging from simple tongue depressors & glucometer strips to large radiology & electronic medical equipments. Individually there are around 5000 different kinds of medical devices and it is not practical to prescribe the local content and percentage of preference in domestic procurement for each medical device. Moreover, DoP needs accurate & reliable data regarding:
- total capacity and production of various categories of medical devices in India,
- the level of competition in the market in different segment of medical devices
- the processes involved in the manufacture of medical devices for prescribing the percentage of minimum local content for each category of medical devices,
The percentage of local content, the manner of calculation of local content and the provision of supplies to be procured from local supplier may be revised after relevant data in this regard becomes available.
However for the time being, based on the present level of the understanding of the medical device market in India and discussion with various industry representatives, DoP, in accordance with Para 5 of PPO, 2017, prescribes the following percentages of minimum local content for various categories of medical devices for preference in public procurement:
Manner of calculation of local content:
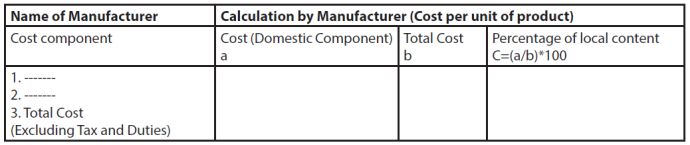
- Local content of Medical Device shall be computed on the basis of the cost of domestic components in the device/service compared to the total cost of the device/ service. The total cost of product shall be the cost incurred for the production of the medical device including direct component i.e. material cost, manpower cost and overhead costs including profit but excluding taxes and duties.
- The determination of local content
cost shall be based on the following:
- in the case of direct component (material), based on the country of origin;
- in the case of manpower, based on domestic manpower
- The calculation of local content of the combination of several kinds of goods shall be based on the ratio of the sum of multiplication of local content of each goods with the acquisition price of each goods to the acquisition price of combination of goods.
- Format of calculation of local content:
Requirement of Purchase Preference
Purchase preference shall be given to local suppliers by all procuring entities as per provisions laid down in para 3 of PPO, 2017. Further, as per the provision of para 3(a) of PPO 2017, in procurement of goods where sufficient local capacity and local competition exist, and estimated value of procurement is upto Rs. 50 Lakhs or less, a list of goods will be issued by this department in due course. Till the time such a list is issued, provision of Para 3(b) and Para 3(c) of PPO, 2017, as applicable, shall apply for all procurements without regards to value of procurement.
Verification of Local Content
- The local supplier, at the time of tender, bidding or solicitation, shall be required to furnish self-certification of local content in the format as contained in the Guidance.
- In case of procurement for a value in excess of Rs. 10 crores, the local supplier shall be required to provide a certificate from a statutory auditor or cost auditor of the company (in case of companies); from a practicing cost accountant and practicing chartered accountant (in respect of suppliers other than companies) giving the percentage of local content.
- In each tender, procuring entity shall clearly mention the details of its competent authority empowered to look into procurement related complaints and the fees for such complaints, relating to implementation of PPO, 2017.
- In case a complaint is received by the procuring entity against the claim of a bidder regarding domestic value addition in medical device, the procuring entity shall have full rights to inspect and examine all the related documents and take a decision. In case any clarification is needed, matter may be referred to DoP to the grievance redressal committee.
- Any complaint referred to the procuring entity shall be submitted along with all necessary documentation in support of the complaint regarding domestic value addition claimed in medical device and shall be disposed of within 4 weeks of the reference by the procuring entity.
- In case, the complaint is referred to DoP by a bidder or procuring entity, the grievance redressal committee to be set up under DoP for the purpose shall dispose-off the complaint.
- In case, the matter is referred to DoP, the grievance redressal committee shall dispose-off the complaint within 4 weeks of its reference and receipt of all documents from the bidder after taking in consideration, the view of the procuring entity. The bidder shall be required to furnish the necessary documentation in support of the local content claimed in medical devices to the grievance redressal committee of DoP within 2 weeks of the reference of the matter. If no information is furnished by the bidder, the grievance redressal committee may take further necessary action, in consultation with procuring entity to establish the bonafides of the claim.
- In case of reference of any complaint to DoP by the concerned bidder, there would be a fee of Rs. 2 Lakh or 1% of the value of the medical devices being procured (subject to a maximum of Rs. 5 Lakh), whichever is higher, to be paid by way of a Demand Draft to be deposited with the procuring entity, along with the complaints by the complainant. In case, the complaint is found to be incorrect, the complaint fee shall be forfeited. In case, the complaint is upheld and found to be substantially correct, deposited fee of the complainant would be refunded without any interest.
Note - All other provisions of PPO, 2017 shall be applicable as such, and shall be adhered to by all procuring agencies for procurement of any medical device. This guideline shall remain applicable for one year from the date of issuance or until further orders are released in this regard.
Footnotes
1 http://pharmaceuticals.gov.in/sites/default/files/Final%20Guidelines.pdf
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

