In Sun Pharmaceutical Industries, Inc. v. Incyte Corporation, on August 22, 2023, the Federal Circuit affirmed a Final Written Decision of the Patent and Trial Appeal Board (the Board) of an inter partes review (IPR) asserting the claims of U.S. Patent No. 9,249,149 (the '149 patent) as obvious under 35 U.S.C. § 103. The central argument was whether Sun's "octo-deuterated" ruxolitinib analog (CTP-543) and "tetra-deuterated" ruxolitinib analogs, arising from claim 7 of the '149 patent, were obvious in light of the prior art references presented by Incyte (Rodgers, Shilling, and the Concert Backgrounder).
Claim 7 recited:
The compound of claim 1, in which the compound is selected from the group consisting of:
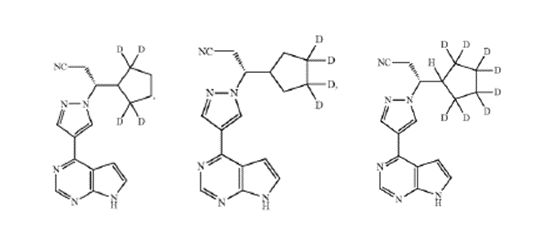
or a pharmaceutically acceptable salt of any of the foregoing.
To determine the obviousness of the chemical compounds, the court used a two-step analysis. First, the court determined whether one of ordinary skill would have selected the prior art compounds as starting points for further development. Second, the court analyzed whether the prior art would have motivated one of ordinary skill in the art to modify the prior art compound to make the claimed compound with a reasonable expectation of success. The present case centers on the second step of the test, as both Sun and Incyte agreed one of ordinary skill in the art would have chosen the prior art as a starting point for further development.
Sun argued the Board erred in three respects, first arguing it had failed to ask whether a person of ordinary skill would have been motivated to deuterate ruxolitinib to alter the compound's pharmacokinetic properties. In deciding whether the Board had erred, the court focused on the cited prior art and testimony from both Sun and Incyte's experts, Dr. Harbeson and Dr. Guengerich respectively. Rodgers (US 5,598,257B) claimed substituted pyrrolo[2,3-b]pyrmidines, including ruxolitinib, while Shilling taught a majority of the ruxolitinib's metabolism occurs on its cyclopentyl rings at the four methylene carbons. Concert disclosed the deuteration of compounds to provide improved safety, tolerability, and efficacy, due to the formation of stronger carbon bonds. Dr. Harbeson testified there were no known relevant differences in selectivity and potency of a compound when deuterated, while Dr. Geungerich's testimony affirmed deuteration's positive effect on a compound's pharmacokinetics. In light of the prior art, expert testimony, and the similarity between the prior art compound and that of the '149 compound, the court concluded one of ordinary skill in the art would have been motivated to deuterate ruxolitinib to alter the compound's pharmacokinetics.
Second, Sun argued the Board had failed to determine whether the person of ordinary skill would have been motivated to make the specific modifications claimed in the '149 patent. Claim 7 of the '149 patent claims the deuteration of the methylene carbons in ruxolitinib's cyclopentyl ring, identified by Schilling as the metabolic hotspots of ruxolitinib. The court concluded that the combination of Concert, teaching that deuterating a compound's metabolic hotspots for improved pharmacokinetics, and Dr. Geungerich's testimony, stating the identified tetra- and octo-deuterated analogs of '149 were the "most reasonable deuterated analogs", would have motivated one of ordinary skill to make the specific modifications claimed in the '149 patent.
Finally, Sun argued the Board had failed to consider whether the person of ordinary skill would have reasonably expected success in modifying ruxolitinib. Specifically, Sun argued that the Board had ignored the "unpredictable" nature of modifying ruxolitinib, relying on a statement made in Concert stating, "the magnitude and nature of the deuterium benefit cannot be predicted a priori." The court determined, however, that the Board had sufficient evidence to conclude modification of ruxolitinib would result in reasonably expected superior pharmacokinetics based on Concert's teachings of deuteration leading to improved safety, tolerability, and efficacy. The court furthered that "some unpredictability" was not enough to rebut a reasonable expectation of success, stating that only a degree of predictability, not absolute predictability, is needed.
Sun attempted to further argue the Board's error by arguing that it had failed to consider two objective indica of nonobviousness: (1) unexpected results and (2) long-felt need. Sun argued the increased therapeutic window and increased half-life in patients who were fast metabolizers of ruxolitinib were unexpected results of the claimed deuteration of ruxolitinib. The court determined that these were not unexpected results because the clinical activity presented by Sun was "merely a difference in degree and not in kind." Sun further argued that the octo-deuterated ruxolitinib analog "satisfied a long-felt need for an FDA-approved, evidence-based alopecia areata treatment." Without determining whether there was an actual need, as stated by Sun, the court solely analyzed whether the long-felt need was satisfied by the '149 compound. The octo-deuterated ruxolitinib analog, however, had not achieved FDA approval, which the court found sufficient to conclude that this long-felt need was not satisfied.
This case is a reminder of what constitutes a motivation to combine prior art, a reasonable expectation of success, and unexpected results when analyzing new chemical compounds. First, when considering whether there is a motivation to combine prior art, the strongest argument to combine will be recognition, expressly or implicitly in the prior art or established scientific principles, that some advantage or an expected benefit would have been produced by the combination. Second, when considering whether there is a reasonable expectation of success, there is not a requirement of absolute predictability. Any degree of predictability may constitute a reasonable expectation of success. Finally, when arguing unexpected results, the result must be one that either markedly differs from the prior art, or differs in kind, not just degree, from the prior art, to be considered truly unexpected.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

