Introduction
The Pharmacovigilance Programme of India (PvPI) is an administrative mechanism that has been developed for monitoring the safety of authorized medicinal products (for human use) and to detect any deviation in their risk-benefit balance.27
The PvPI mechanism rests on continuous communication to stakeholders and risk management of Adverse Drug Reactions (ADRs). Stakeholders in the PvPI system include healthcare bodies and professionals, manufacturers of drugs (i.e. Market Authorization Holders/MAHs), Adverse Drug Reaction (ADR) Monitoring Centres (AMCs), regulatory bodies such as the Central Drugs Standard Control Organization (CDSCO) and ADR reporting-cum-analysis bodies within the National Coordinating Centre (NCC)- Indian Pharmacopeia Commission (IPC).
This article attempts to provide an outline of the system of pharmacovigilance in India.
Authorities and their respective roles
A. CDSCO
- Taking regulatory decision on the basis of recommendations of Signal Review Panel (SRP)28 and the Steering Committee of NCC –PvPI at IPC,
- Decisions on the basis of scientific evaluation of Periodic Safety Update Report (PSUR) data, and
- Utilization of evidence-based information to initiate appropriate regulatory decision such as changing/ updating package-insert29, issuance of Drug Alerts30, banning of drug, etc.
B. State Drug Regulatory Authority
- Monitor ADRs as per Para 28, Schedule M31 of the Act (based on information received from MAHs) and the same is also forwarded to NCC-PvPI.32
C. Indian Pharmacopoeia Commission
IPC is the nodal agency which is functioning as NCC of PvPI program, supervising and regulating the PvPI programme (see, for details Ch. 1 Guidance Document). It is also responsible for coordinating with WHO-Uppasla Monitoring Centre, Sweden. The following bodies work under NCC:
- AMCs are responsible for collection and reporting of ADRs.
- The Steering Committee supervises and provides overall guidance for the PvPI programme. Further, the decisions of the Steering Committee are presented to the Drugs Technical Advisory Board (DTAB) by CDSCO.33
- Working group reviews and monitors major technical issues related to establishment and implementation of the programme. It also provides technical inputs to CDSCO for appropriate regulatory intervention/actions.
- Quality Review Panel reviews quality, prepares causality assessment reports and ensures completeness of Individual Case Safety Reports (ICSRs).
- Signal Review Panel is responsible to assess the database for the occurrence of 'signals' of possible repercussions on public health, drug regulation and science.
Steps in PvPI programme
Step 1: ADR Reporting
- Who can report: All healthcare professionals (clinicians, dentists, pharmacists and nurses) and consumers may report ADRs. Pharmaceutical companies can also report ICSRs/ PSURs-specifics for their products. AMCs (which are basically hospitals or medical colleges) are responsible for continuous monitoring of drugs; all reports received through AMCs are entered into a digital software known as 'Vigiflow' which is maintained by the World Health Organisation (WHO).
- How to Report: Different ADR forms are available at http://www.ipc.gov.in/PvPI/adr.html for reporting adverse drug reactions.
- Whom to report: Such ADR forms can be submitted to AMCs or directly to NCC. One can also report through NCC-PvPI helpline - 1800 180 3024.
- The above data collected is used for signal generation, risk management, drug regulation and educational purposes.
Step 2: Assessment of Adverse Event
- Causality Assessment involves the evaluation of likelihood that a medicine was the causative agent of an observed adverse event. AMCs are responsible for causality assessment reports which shall be reviewed at NCC. NCC may further route these communications to CDSCO. (For details see, Ch. 6 Guidance Document)
- Signal Detection: If there is any causal relationship between an adverse event and a drug and such relationship was previously unknown or incompletely documented, a 'signal' is generated.34 More than a single report is required to generate the signal depending upon the seriousness of event and the quality of information.
- NCC performs the identification and initial review of a signal, the results of which are submitted to the SRP. SRP reviews the report against the following parameters - quality, content and completeness of data.35
- The information generated by NCC on the basis of ADR reports assists in continuous assessment of the benefit-risk ratio of medicines. The information is submitted to the Steering Committee of PvPI constituted by the Ministry of Health & Family Welfare (MOHFW). The Committee is entrusted with the responsibility to review the data and suggest any intervention that may be required.36
- NCC will communicate the findings of aforementioned review and also the metrics related to ADR data to CDSCO through a monthly report.
Step 3: Risk Management and Communication
- The scientific data collected and analysed in Step 2 is forwarded by NCC to the CDSCO for appropriate regulatory actions.37
- CDSCO is also responsible for giving
directions to the concerned state Licensing Authorities for taking
appropriate actions in the form of change in label/package inserts
or recall in relation to the drugs which have been identified as
spurious, sub-standard and injurious to human health by NCC in Step
2.38 The flow of aforementioned regulatory directives is
illustrated below:
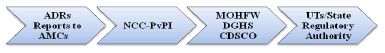
- Communications to all PvPI
stakeholders will be the primary responsibility of the CDSCO-HQ.
The linkages outlined in the flowchart below39 ensure
that pharmacovigilance information is well-circulated and all
stakeholders are kept in the loop.

Figure 1: Communication Flow in PvPI Programme
Conclusion
Having commenced in 2010, the PvPI programme now has 250 AMCs working across India.40 During the period 2011-201641, it received 1,81,656 ADR reports from all sources.42 In the period 2015-16, the SRP made eleven recommendations regarding change in label for investigational drugs to CDSCO - out of which three recommendations got approved.43 The work of PvPI has been duly recognised by WHO. It has been made WHO-Collaborating Centre for Public Health Programmes and Regulatory Services.44 The WHO-NRA assessment awarded highest maturity level – 4 out of 5, to PvPI.45
Every drug has benefits and risks associated with its use. It is only when the benefits outweigh the risk that the drug is considered safe for human use. The process of benefit-risk analysis is a continuous process. The current PvPI system is an attempt to bring uniformity in post-marketing surveillance of a drug, creating a single-window collection and analysis of ADRs and bringing the current regime at par with global WHO standards.
Footnotes
27. http://www.ipc.gov.in/PvPI/about.html
28. A 'signal', as defined by the World Health Organization, is reported information of preliminary nature that seeks to establish a causal relationship between an adverse reaction and the use of a drug, in the document titled 'Signal Review Panel under PvPI' published by IPC.
29. DCGI letter to State Drug Controllers dated 12th May, 2015 directing manufacturers of the drug Carbamezepine to effect changes in safety label based on recommendations of the SRP and Subject Expert Committee.
30. PvPI releases monthly drug alerts based on preliminary assessment of ADRs to direct healthcare professionals and consumers to closely study the effect of such drugs and report further ADRs, if any.
31. This provision makes it an obligation of the MAH to report ADRs to the State Licensing Authority as a part of 'Good Manufacturing Practices'.
32. Pharmacovigilance Guidance Document for Market Authorization Holders of Pharmaceutical Products published by IPC, January 2018, p. 2-3 (Guidance Document). Also see, PvPI Newsletter, Vol. 6 Issue 16 available at: http://www.ipc.gov.in/PvPI/newsletter/Newsletter%20Vol%206%20Issue%2016%202016%20PDF.pdf
33. Process and Procedures as per the 'Standard Operating Procedure for Communications in Pharmacovigilance Programme of India' dated 22nd February, 2011, p. 4-5
34. See Generally, Ch. 7, Guidance Document, supra note 6.
35. Generally see, PvPI performance report available at: http://www.ipc.gov.in/PvPI/pub/Pharmacovigilance%20Programme%20of%20India%20Annual%20performance%20Report%202014-2015.pdf
36. Advice on Reporting as stipulated in the Suspected Adverse Drug Reaction Reporting Form, Version 1.2, IPC (available at https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=MzAz)
37. See generally, Indian Pharmacopoeia notification ref no.: IPC/NCC-PvPI/ SRP/2016-17 dated 14.12.2016 in which PVPI recommend that regulatory actions be undertaken for 24 drugs. The copy of the said notification is available at: http://www.cdsco.nic.in/writereaddata/PvPI%20recommendations.pdf
38. See generally, CDSCO notification ref. no. File no. 4-01/2015-DC (Misc.82) dated 23.12.2015 CDSCO available at: http://www.ipc.gov.in/PvPI/das/Regulatory%20Pharmacovigilance%20-Potential%20signal%20and%20Recommendation%20for%20the%20label%20change%20of%20certain%20medicinal%20product%20marketed%20in%20India%20-%20Reg.pdf
39. This diagram has been taken from the Guidance Document for Spontaneous Adverse Drug Reaction Reporting of May, 2014 pblished by the IPC, p. 6
40. www.ipc.gov.in/PvPi/adr/ADR.pdf
41. Data for 2016-17, 2017-18 is not been available.
42. PvPI, Annual Performance Report, 2015-16 http://www.ipc.gov.in/PvPI/pub/Pharmacovigilance%20Programme%20of%20India%20Annual%20 performance%20Report%202015-2016.pdf
43. Id.
44. http://www.ipc.gov.in/PvPI/newsletter/Newsletter%20Vol%207%20Issue%2021%202017%20PDF.pdf
45. Id.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

