We've written previously about the the 2014 final regulation issued by the Obama administration making significant updates to the requirements for the qualities of settings eligible for reimbursement for Medicaid home-and-community-based services (HCBS) provided under sections 1915(c), 1915(i) and 1915(k) of the Medicaid statute. With the deadline for full compliance nearing (HCBS providers must comply with the new requirements by March 17, 2022, or they will not be able to receive Medicaid payment for HCBS) — the Medicaid and CHIP Payment and Access Commission (MACPAC) has now issued a report detailing:
- A background on the 2014 HCBS rule; and
- A review of stakeholder perspectives on the implementation process, so far.
Below we briefly review and remind our readers of the import of the 2014 final HCBS rule, and then dive into the interesting perspectives shared with MACPAC from states, providers, and beneficiaries as the deadline for compliance nears.
A Brief Review of the 2014 Final Rule
On January 10, 2014, the Obama Administration published in final rulemaking a significant update to the program requirements for HCBS services. The rulemaking emerged in partial response to a 1999 Supreme Court Decision (Olmstead v. L.C., 527 U.S. 581) in which the Supreme Court affirmed a state's obligations to provide covered program services to eligible individuals with disabilities in the most integrated setting appropriate to their needs. As a result of the 2014 Final Rule, CMS adopted new minimum standards that all HCBS setting must meet, including that the setting:
- The setting is integrated in and supports full access to the greater community;
- Is selected by the individual from among setting options;
- Ensures individual rights of privacy, dignity and respect, and freedom from coercion and restraint;
- Optimizes autonomy and independence in making life choices; and
- Facilitates choice regarding services and who provides them.
While explicitly excluding certain settings as permissible for HCBS services (for example, nursing facilities and institutions for mental disease), the final rule also identified other settings that are presumed to have "institutional qualities," and thus do not meet the threshold for Medicaid HCBS (for example, those that have the effect of isolating individuals from the broader community of individuals not receiving Medicaid-funded HCBS).
On March 22, 2019, CMS published an updated letter to State Medicaid Directors and a new guidance document regarding the Home and Community-Based Services (HCBS) waiver program. The letter revises previous guidance that CMS had provided to states on "Settings that have the effect of isolating individuals receiving HCBS from the broader community" for purposes of receiving Federal funding for services provided under a HCBS waiver.
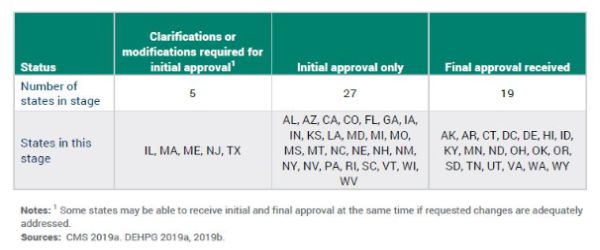
As noted above, HCBS providers must comply with the new requirements by March 17, 2022, or they will not be able to receive Medicaid payment for HCBS. The following table, published by MACPAC, shows the current status of statewide transition plan approval, as of December 2019.
Stakeholder Perspectives Shared with MACPAC on the HCBS Final Rule Implementation Process
By way of background, in response to the looming March 2022 deadline for compliance with the 2014 HCBS final rule, MACPAC officials interviewed federal and state officials, beneficiary advocates, and providers between October 2018 and July 2019 to solicit their feedback on the final rule implementation process. MACPAC generally broke their findings into: (1) Federal activities; (2) State perspectives; and (3) provider and beneficiary comments.
With regard to Federal activities, MACPAC spoke with officials from both CMS and the Administration for Community Living (ACL) who are working with states to come into compliance. Both CMS and ACL officials have provided guidance for compliance through webinars, teleconferences, and guidance documents. MACPAC staff noted that most of this guidance has thus far been focused on residential settings (i.e. intentional communities) vs. non-residential settings (i.e. employment supported settings). CMS officials noted they may provide more guidance on non-residential settings in the future.
States were generally supportive of the concept of the rule – but noted the piecemeal implementation and rollout of guidance created a difficult environment in which to operate. States gave examples of needing to re-do processes or backtrack on implementation following the release of much-delayed guidance from CMS, or slight policy shifts. There were evident differences in those states with approved plans already in place (early engagement with the provider and beneficiary communities) and those without. A number of states raised the issue of conflicts between state law and CMS' guidance, forcing the state to treat whole classes of providers as subject to the heightened scrutiny standard.
Providers, for their part, expressed the most concern about the costs associated with compliance with the final rule. In particular, providers noted that existing payment rates do not account for the costs needed to transform or otherwise meet the new HCBS setting rules. Provider groups also made the interesting comment that the rule tries to fit too many disparate populations into a single standard. As an example, providers note that while phasing out isolating settings may be a key priority for meeting the needs of individuals with intellectual disabilities (ID) and developmental disabilities (DD), this may be less of a concern for meeting the needs of older adults.
Finally, the beneficiary advocate community expressed a number of concerns related to both process and substance. Beneficiary advocates complained about a lack of transparency in the guidance process, as well as difficulty in deciphering the guidance documents when they are finally located. Beneficiary advocates also expressed concern about the extent to which CMS has, in its most recent March 2019 guidance, delegated too much responsibility to states, perhaps weakening Federal oversight standards.
We will continue to track the statute of implementation and compliance as the Federal deadline nears.
To view Foley Hoag's Medicaid and the Law blog please click here
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

