The Federal Circuit recently upheld the Patent Office's decision to reject claims in four separate reexamination cases due to obviousness-type double patenting (ODP). In re Cellect, LLC, Appeal Nos. 22-1293, -1294, -1295, -1296 (Fed. Cir. Aug. 28, 2023). This decision is important because it expands ODP, a doctrine judges developed long ago, when patents received a term of 17 years from the date of issue, rather than 20 years from the date of filing. Unless overturned, this decision will significantly affect patent families that have patents with different expiration dates. This post explains some of the decision's consequences and explores potential patent prosecution strategies.
What transpired in Cellect was not entirely surprising after the court's 2014 decision in Gilead Sciences, Inc. v. Natco Pharma Ltd., 753 F.3d 1208 (Fed. Cir. 2014). In that case, Gilead Sciences sequentially filed separate patent applications, leading to patents with different expiration dates, claiming inventions that were obvious variants of each other. The relevant facts are illustrated below.
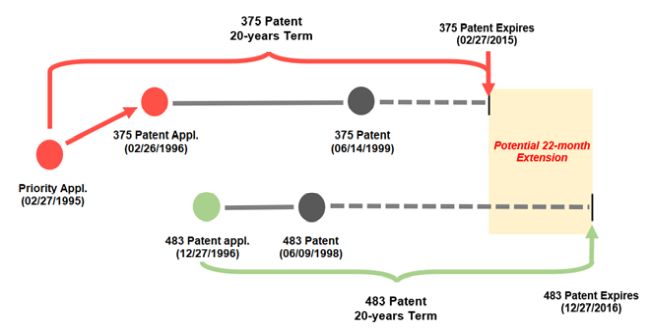
Unrelated Patents in Gilead Sciences
On these facts, the Federal Circuit concluded that the earlier-expiring '375 patent can serve as an ODP reference against the family-unrelated and later-expiring '483 patent, despite the '375 patent being issued after the '483 patent. The Federal Circuit explained that it's the expiration dates, not the issue dates, that dictate whether ODP may apply. Id. at 1215. Through that explanation, Gilead Sciences introduced new law. Many hoped, however, the holding in Gilead Sciences would be limited to its uncommon facts.
More commonly, ODP involves a group of patents from the same family, all sharing the same effective filing and expiration dates, except for any patent term adjustments (PTA). For example, in a series of related patent applications, the first one submitted, or the "parent," often endures the lengthiest prosecution and Patent Office delays, thereby accruing PTA. Subsequent "child" applications benefit from the proceedings of the parent application, resulting in shorter prosecution and, consequently, little to no PTA. If a child application is a continuation of the parent (rather than a divisional), it may encounter an inconsequential ODP rejection over the parent, which by then has likely issued as a patent. This rejection can be bypassed with a terminal disclaimer. Eventually, the child application issues with no PTA, and possibly a terminal disclaimer over the term-adjusted parent.

Parent with PTA and its Child
The child patent expires 20-years from the filing date of the earliest benefit-claimed, non-provisional application (here, the parent's filing date). The child does not receive the PTA the parent earned; instead, the child's term is simply measured from the parent's filing date. See 35 USC § 154(a)(2). Some district courts presented with these facts applied Gilead Sciences to conclude that the (later-expiring) term-adjusted parent is vulnerable to invalidity for ODP over claims in the later-issuing child. Specifically, these courts concluded that the earlier-expiring child can serve as an ODP reference against the later-expiring parent where the child issued—as it often will—after the parent.* These cases never reached the Federal Circuit for review.
Several years after Gilead Sciences, Cellect accused a third party of infringing a family of patents, each patent expiring on different dates due to PTA that all but one received. The third party persuaded the Patent Office to reexamine the patents. The reexamination examiner applied the judicially created doctrine of ODP to reject the challenged claims. The Patent Trial and Appeal Board eventually issued final decisions affirming the rejections. Cellect appealed the Board's decisions to the Federal Circuit.
On appeal, the court stated that ODP exists when a claim in a later-expiring patent would have been obvious over a claim in an earlier-expiring patent owned by the same entity. Cellect, Slip Op. at 15. The court cited no authority for this statement. The statement is rooted, however, in Gilead Sciences, which explained that the ODP "doctrine prohibits an inventor from extending his right to exclude through claims in a later-expiring patent that are not patentably distinct from the claims of the inventor's earlier-expiring patent." Gilead Scis., 753 F.3d at 1210. Gilead Sciences did not, however, involve family-related patents that received PTA. And the court there made that statement in the context of peculiar circumstances. Regardless, in Cellect the court decided that which patent is "later" depends upon the PTA received. In Cellect, this meant that claims in a reference patent that received no PTA—the '036 patent—rendered obvious claims in each of two other patents, the '369 and '626 patents, that received 45 and 59 days of PTA, respectively. In turn, claims in those two patents rendered obvious claims in two other patents that had received even more PTA, the '621 and '742 patents.
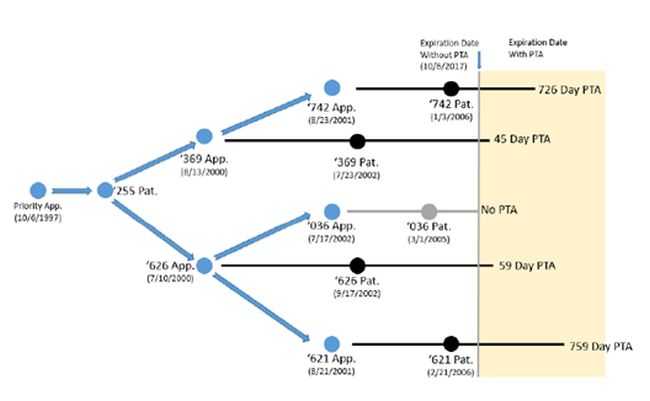
Cellect Patent Family
(Image from Cellect LLC Principal Brief, page 12 (May 16, 2022)
This application of ODP is visually striking, yet also unsurprising given how some district courts had already expansively applied Gilead Sciences.* Specifically, as in those district court decisions, the Patent Office concluded (and the court affirmed) that claims in the later-expiring and term-adjusted parent, the '626 patent, are unpatentable for ODP over claims in the later-issuing and earlier-expiring child, the '036 patent. Indeed, the issuance of the child '036 patent effectively invalidated the claims in the '626 parent patent.
Even more importantly, the court "conclude[d] that ODP for a patent that has received PTA, regardless whether or not a terminal disclaimer is required or has been filed, must be based on the expiration date of the patent after PTA has been added." Cellect, Slip Op. 21 (emphasis added). That conclusion seems to counsel in favor of filing preemptive terminal disclaimers before the reference patent has expired, e.g., filing terminal disclaimers even when the examiner hasn't issued an ODP rejection. Why? Absent the terminal disclaimer, the claims might be invalid upon issuance or, in some instances, upon the reference patent's issuance. The patentee may not learn this until adjudication or reexamination, years later. But filing a terminal disclaimer then may be too late if the patentee is interested in collecting past damages and/or too late if the reference patent has expired.
The examiner never issued ODP rejections during original prosecution of the Cellect patents. Unsurprisingly, no terminal disclaimers were filed. Eventually, the '036 patent expired. The claims determined during reexamination to be unpatentable due to ODP would have been enforceable at least during the 20-year statutory term of the '036 patent had terminal disclaimers been filed during that term.
[C]ourts should be reluctant to create or expand judge-made exceptions to statutory grants.
To patent owners, there are inequities in a child knocking out the term-adjusted parent:
- The holding in Gilead Sciences should have been confined to its uncommon facts where a family-unrelated, earlier-expiring but later-issued patent was applied as an ODP reference to invalidate a commonly owned patent. After all, the court specifically "h[e]ld that an earlier-expiring patent can qualify as an obviousness-type double patenting reference for a later-expiring patent under the circumstances here." Gilead Sci., 753 F.3d at 1217 (emphasis added); see also, id. at 1218 (Rader, C.J., dissenting) (citing Supreme Court cases for the proposition that "courts should be reluctant to create or expand judge-made exceptions to statutory grants").
- The statutory PTA awarded the parent compensates the patent owner for term lost due to prosecution delays that are solely the Patent Office's fault, not the gamesmanship the Federal Circuit cautioned against in Gilead Sciences. In Cellect, the court dismissed the patent owner's arguments that it had not engaged in gamesmanship to obtain term-adjusted patents. Cellect, Slip Op. at 23.
- When a parent issues with PTA, the public has notice of its scope and term of exclusivity. The public is not harmed when the patent owner is later issued a child patent that expires no later than its parent. Expanding Gilead Sciences to permit a child to knock out a term-adjusted parent contravenes the notice function of patents. This is because the parent's expiration date suddenly becomes uncertain whenever the patent owner has a child application pending. Could Congress have intended that, despite statutory provisions authorizing child applications and PTA?
And several amici informed the court of other maladies that would accompany the court's affirmance in Cellect.
Cellect is a gift, however, to those facing allegations of infringing term-adjusted patents with family members that did not receive PTA. To them, Cellect presents no inequities because the patent owner controlled the presentation of claims throughout its various applications. The patent owner theoretically could have presented all obvious variants in a single patent that receives PTA, rather than spreading them out in other family-related patents expiring earlier.
So, after Cellect, what might owners of patent families consider doing? The common owner of two family-related patents—having different expiration dates due to PTA—may need to file a terminal disclaimer in the target and reference patents to obviate ODP or overcome ODP on its merits by argument or by amendment. Each option presents challenges, and some might be difficult to overcome. Filing a terminal disclaimer in the term-adjusted parent will naturally eliminate its PTA. But a terminal disclaimer may be necessary to ensure the parent patent remains valid from the time the child patent is issued until the parent's original (non-PTA) expiration date. (The statute, 35 USC § 121, provides a safe harbor from ODP attacks where one patent is a divisional of the other.)
The patent owner might consider reissuing the parent patent to address potential ODP that may arise from a child patent issuing. To do this, however, there needs to be a correctable error in the patent—like flaws in the specification or claims that render the patent inoperative or invalid (as stated in 35 USC § 251(a)). As of now, no case has addressed whether a post-issuance ODP issue is such an error. The error in the parent, an adversary might argue, arose only after the earlier-expiring child issued. Even if that is a correctable error, an application to reissue invites new examination that may necessitate claim amendments, which, in turn, may implicate intervening rights.
Overcoming ODP on the merits solely with arguments, without making any amendments, can be challenging. For example, if the term-adjusted parent issues with a genus claim, and the child is prosecuted to obtain a species claim, the species claim (in the earlier-expiring child) will anticipate the genus claim. Absent a terminal disclaimer filed in the parent, the parent is not enforceable—even during that commonly-shared portion of the term defined as 20-years-from-filing. Cellect, Slip Op. at 25. The same result likely ensues where the claims in the two patents have overlapping scope and are not necessarily in a genus-species relationship.
In prosecuting an application that may enjoy PTA, it is important to obtain as many claims as possible to obviate the ODP potential (of Cellect) accompanying claims that later issue in a continuation (child). In the two examples above, the claims should likely be prosecuted together. Once the term-adjusted patent issues, any earlier-expiring patent should be prosecuted with claims that do not anticipate or render obvious any claim of the term-adjusted patent. In a situation where the term-adjusted parent issues with a species claim and the child is prosecuted to obtain a genus claim, beware of the potential that the genus claim, combined with other prior art, might render obvious the species claim.
Thus, after Cellect and until or unless it is overruled, the patent owner may consider filing terminal disclaimers, abandoning the child application(s), and reissuing the parent to include allowable claims of the child. Alternatively, the patent owner may consider and try prosecuting a single patent with all possible claims to the same invention, including obvious variants that it might otherwise present in one or more continuation applications. Of course, that's easier said than done. Where the Patent Office is delinquent in examining a patent application and PTA is important to the patent owner, no strategy may offer complete protection against the potential pitfalls of the judge-made law introduced long ago and expanded in Gilead Sciences and Cellect.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

